Publications
(22) Afzaal M. Shareef, Guizi Chen, Claiborne M. Christian, Benjamin Freeman, Edgar Africano Camargo, Jude N. Ubi, Shuaihua Gao, Dawn LaCoste, Victoria P. Belancio*. Functional analyses identify intramolecular interactions within the L1 ORF2p required for its enzymatic activities. In revision.
(21) Edgar Africano Camargo, Hanzi Gao, Jude N Ubi, Xiuyuan Duan, Xiaolin Tian, Haiteng Deng, GuojunZheng*, and Shuaihua Gao*. Hydrogen-Deuterium Exchange Reveals Catalytically Linked Protein Flexibility in Myoglobin-Mediated Intramolecular C(sp³)-H Activation. Protein Sci. 2026;35(1):e70410. doi: 10.1002/pro.70410. PMID: 41427743; PMCID: PMC12720788. https://onlinelibrary.wiley.com/doi/10.1002/pro.70410
(20) Hanzi Gao, Edgar Africano Camargo, Jude N Ubi, Xiuyuan Duan, Guojun Zheng, Shuaihua Gao* and Qipeng Yuan. Stereoselective construction of chiral flavonoids via enzymatic intramolecular C(sp3)-H activation. Org. Chem. Front., 2025. https://pubs.rsc.org/en/Content/ArticleLanding/2025/QO/D4QO02379J
(19) Shuaihua Gao, Xin Ting Wu, Wenju Zhang, Tyre Richardson, Samuel L. Barrow, Christian A. Thompson-Kucera, Anthony T. Iavarone, and Judith P. Klinman. Temporal Resolution of Activity-Related Nanosecond Solvation Dynamics in the TIM Barrel Enzyme Murine Adenosine Deaminase. ACS Catalysis, 2024, 14, 4554–4567. https://pubs.acs.org/doi/10.1021/acscatal.3c02687
Before Tulane
(18) Gao, S and Klinman, J. P., Functional roles of enzyme dynamics in accelerating active site chemistry: emerging techniques and changing concepts. Current Opinion in Structural Biology, 2022, 75, 102434. (Invited Review paper). https://www.sciencedirect.com/science/article/pii/S0959440X22001130?via%3Dihub
(17) Gao, S.; Zhang, W.; Barrow, S. L.; Iavarone, A. T.; Klinman, J. P., Temperature-dependent hydrogen deuterium exchange shows impact of analog binding on adenosine deaminase flexibility but not embedded thermal networks. Journal of Biological Chemistry, 2022, 298(9), 102350. https://www.sciencedirect.com/science/article/pii/S0021925822007931?via%3Dihub
(16) Gao, S.; Thompson, E. J.; Barrow, S. L.; Zhang, W.; Iavarone, A. T.; Klinman, J. P., Hydrogen–Deuterium Exchange within adenosine deaminase, a TIM barrel hydrolase, identifies networks for thermal activation of catalysis. Journal of the American Chemical Society 2020, 142 (47), 19936-19949. https://pubs.acs.org/doi/10.1021/jacs.0c07866
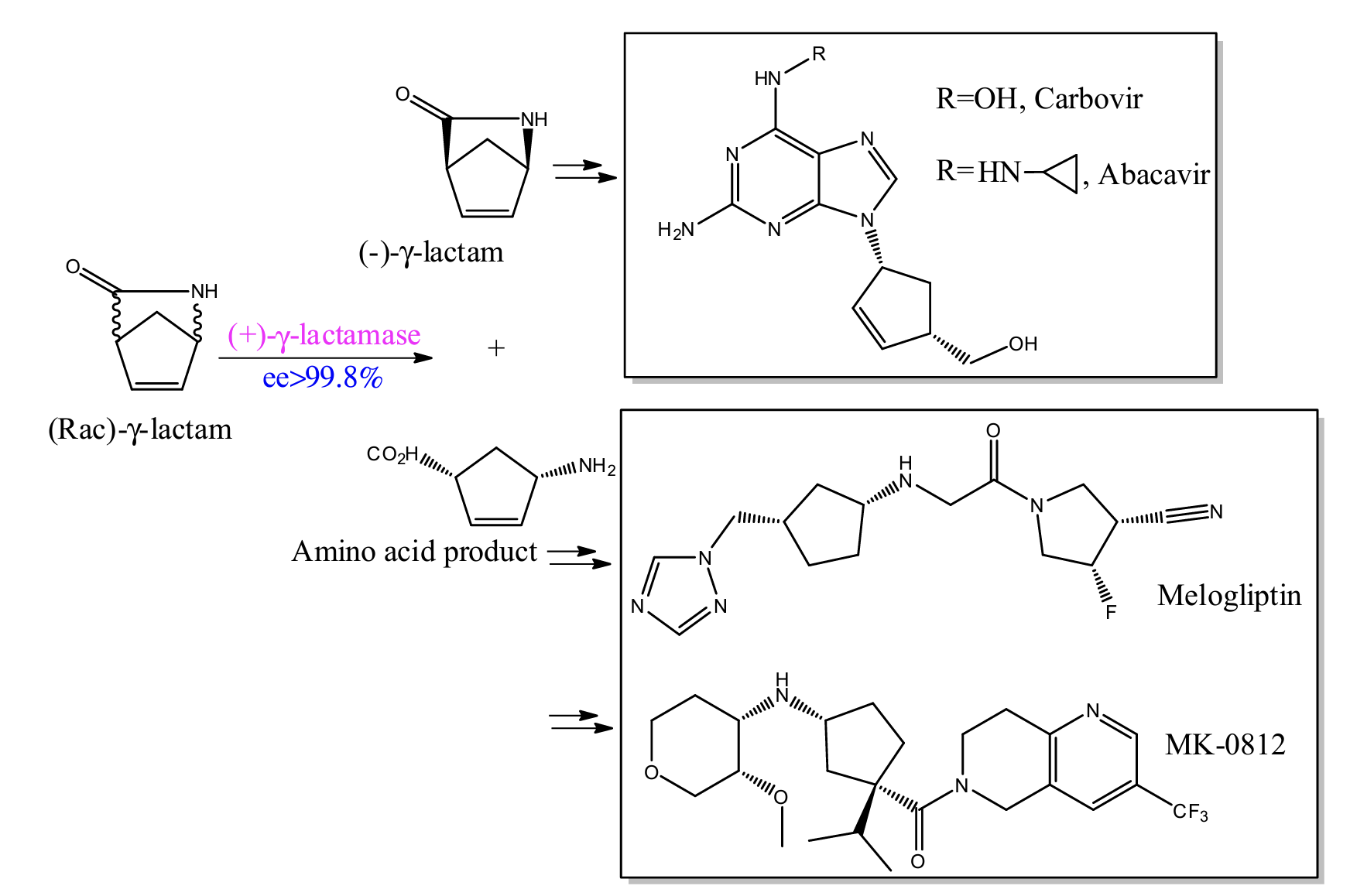
(15) Zhang, J.; Balsbaugh, J. L.; Gao, S.; Ahn, N. G.; Klinman, J. P., Hydrogen deuterium exchange defines catalytically linked regions of protein flexibility in the catechol O-methyltransferase reaction. Proceedings of the National Academy of Sciences 2020, 117 (20), 10797-10805. https://www.pnas.org/doi/10.1073/pnas.1917219117?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
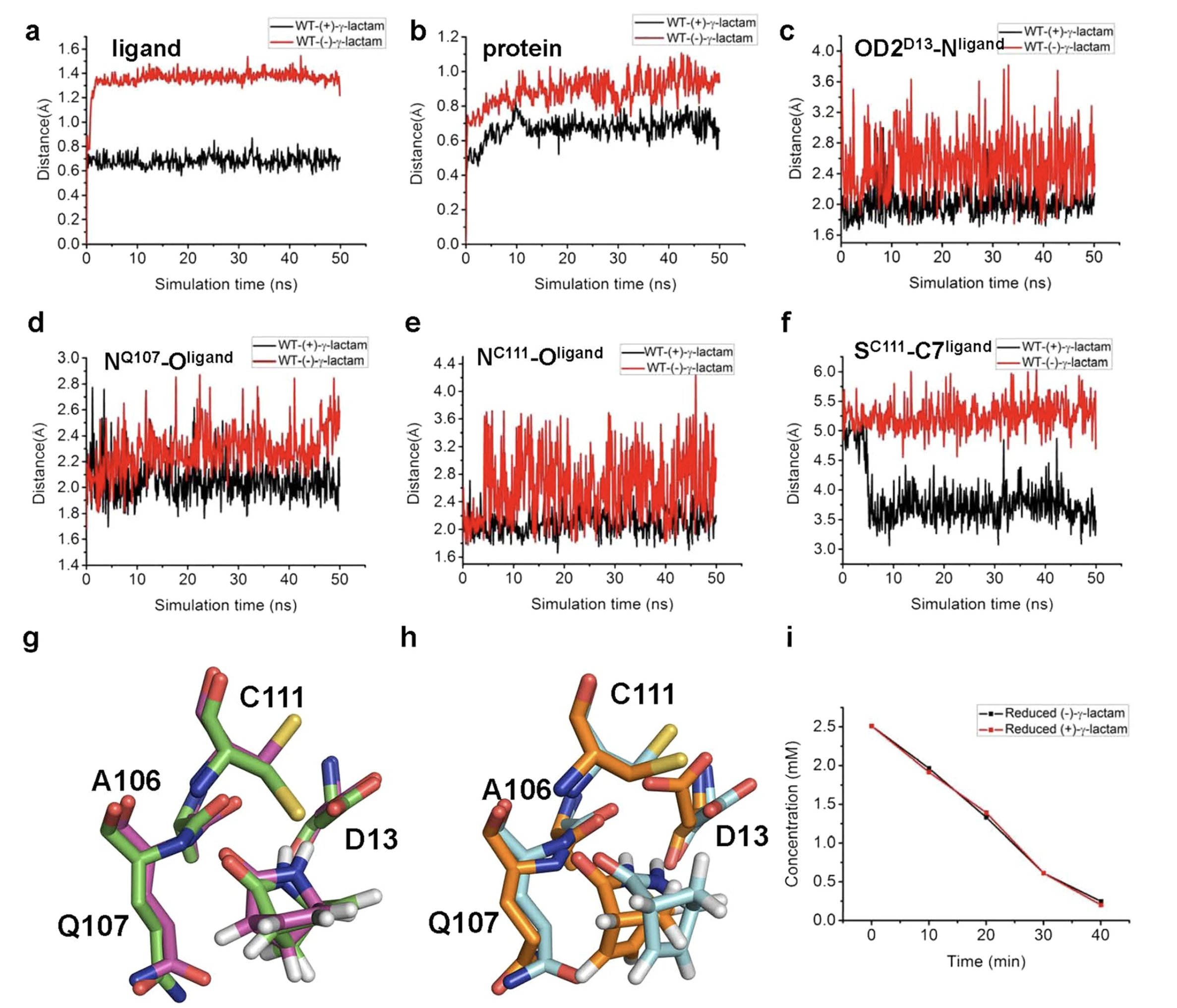
(14) Gao, S.; Lu, Y.; Li, Y.; Huang, R.; Zheng, G., Enhancement in the catalytic activity of Sulfolobus solfataricus P2 (+)-γ-lactamase by semi-rational design with the aid of a newly established high-throughput screening method. Applied microbiology and biotechnology 2019, 103 (1), 251-263. https://link.springer.com/article/10.1007/s00253-018-9428-0
(13) Gao, S.; Zhu, S.; Huang, R.; Li, H.; Wang, H.; Zheng, G., Engineering the Enantioselectivity and Thermostability of a (+)-γ-Lactamase from Microbacterium hydrocarbonoxydans for Kinetic Resolution of Vince Lactam (2-Azabicyclo [2.2. 1] hept-5-en-3-one). Applied and environmental microbiology 2018, 84 (1). https://journals.asm.org/doi/10.1128/AEM.01780-17?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
(12) Gao, S.; Zhou, Y.; Zhang, W.; Wang, W.; Yu, Y.; Mu, Y.; Wang, H.; Gong, X.; Zheng, G.; Feng, Y., Structural insights into the γ-lactamase activity and substrate enantioselectivity of an isochorismatase-like hydrolase from Microbacterium hydrocarbonoxydans. Scientific reports 2017, 7, 44542. https://www.nature.com/articles/srep44542
(11) Gao, S.; Huang, R.; Zhu, S.; Li, H.; Zheng, G., Identification and characterization of a novel (+)-γ-lactamase from Microbacterium hydrocarbonoxydans. Applied microbiology and biotechnology 2016, 100 (22), 9543-9553. https://link.springer.com/article/10.1007/s00253-016-7643-0
(10) Gao, S.; Su, Y.; Zhao, L.; Li, G.; Zheng, G., Characterization of a (R)-selective amine transaminase from Fusarium oxysporum. Process Biochemistry 2017, 63, 130-136. https://www.sciencedirect.com/science/article/pii/S1359511317306712
(9) Gao, S.; Zhu, S.; Huang, R.; Lu, Y.; Zheng, G., Efficient synthesis of the intermediate of abacavir and carbovir using a novel (+)-γ-lactamase as a catalyst. Bioorganic & Medicinal Chemistry Letters 2015, 25 (18), 3878-3881. https://www.sciencedirect.com/science/article/pii/S0960894X15007581
(8) Chen, Y.; Gao, F.; Zheng, G.; Gao, S.,* Enantioselective synthesis of a chiral intermediate of himbacine analogs by Burkholderia cepacia lipase A. Biotechnology Letters 2020, 42 (12), 2643-2651. https://link.springer.com/article/10.1007/s10529-020-02969-z
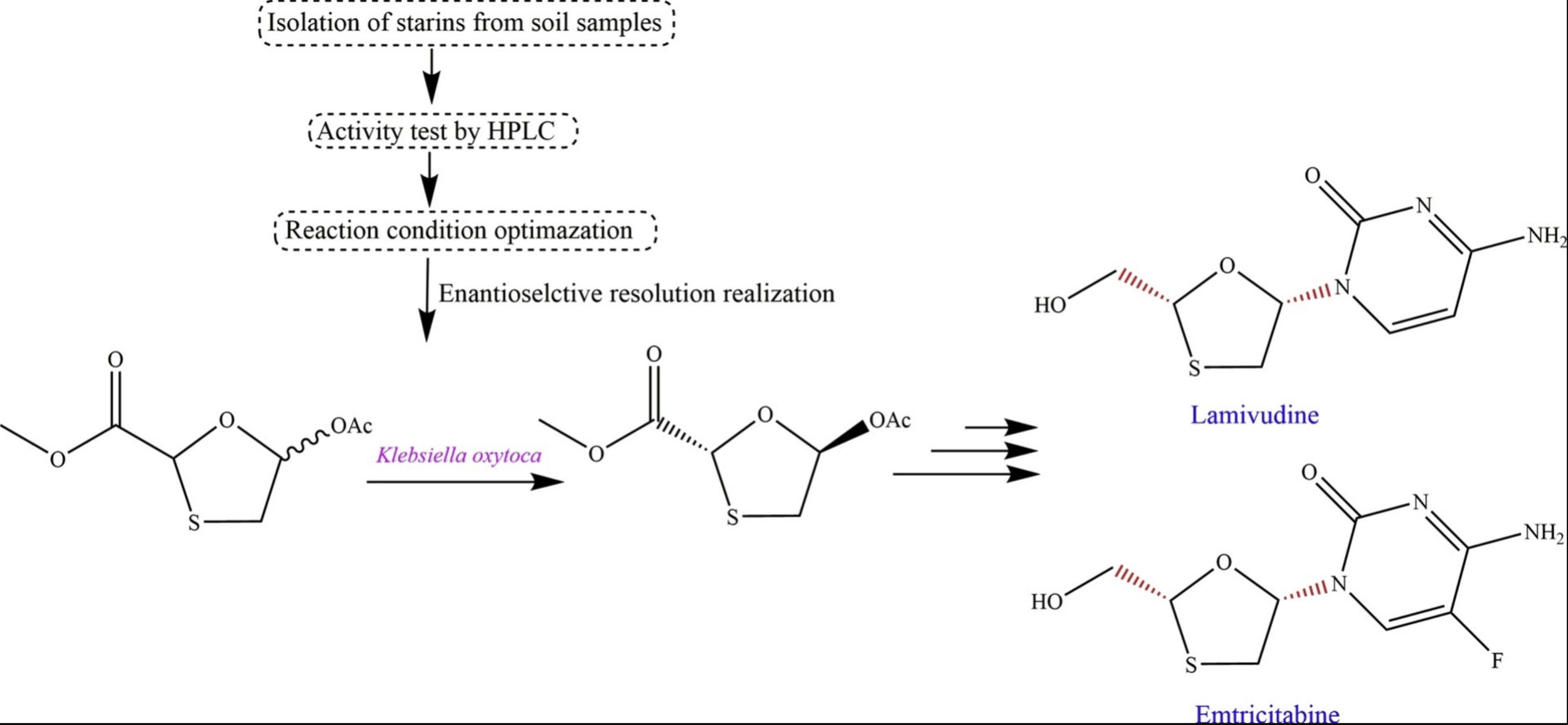
(7) Chen, Y.; Zhang, X.; Zheng, G.; Gao, S.,* Preparation of the enantiomerically enriched precursor of lamivudine (3TC™) via asymmetric catalysis mediated by Klebsiella oxytoca. Process Biochemistry 2019, 81, 77-84. https://www.sciencedirect.com/science/article/pii/S1359511319300790
(6) Li, H.; Gao, S.; Qiu, Y.; Liang, C.; Zhu, S.; Zheng, G., Genome mining integrating semi-rational protein engineering and nanoreactor design: roadmap for a robust biocatalyst for industrial resolution of Vince lactam. Applied Microbiology and Biotechnology 2020, 104 (3), 1109-1123. https://link.springer.com/article/10.1007/s00253-019-10275-6
(5) Shen, X.; Zhou, D.; Lin, Y.; Wang, J.; Gao, S.; Kandavelu, P.; Zhang, H.; Zhang, R.; Wang, B.-C.; Rose, J., Structural Insights into Catalytic Versatility of the Flavin-dependent Hydroxylase (HpaB) from Escherichia coli. Scientific reports 2019, 9 (1), 7087. https://www.nature.com/articles/s41598-019-43577-w
(4) Su, Y.; Gao, S.; Li, H.; Zheng, G., Enantioselective resolution of γ-lactam utilizing a novel (+)-γ-lactamase from Bacillus thuringiensis. Process Biochemistry 2018, 72, 96-104. https://www.sciencedirect.com/science/article/pii/S1359511318305890
(3) Zhu, S.; Huang, R.; Gao, S.; Li, X.; Zheng, G., Discovery and characterization of a second extremely thermostable (+)-γ-lactamase from Sulfolobus solfataricus P2. Journal of bioscience and bioengineering 2016, 121 (5), 484-490. https://www.sciencedirect.com/science/article/pii/S1389172315003710
(2) Ren, L.; Zhu, S.; Shi, Y.; Gao, S.; Zheng, G., Enantioselective resolution of γ-lactam by a novel thermostable type II (+)-γ-lactamase from the hyperthermophilic archaeon Aeropyrum pernix. Applied biochemistry and biotechnology 2015, 176 (1), 170-184. https://link.springer.com/article/10.1007/s12010-015-1565-7
(1) Zhu, S.; Gong, C.; Song, D.; Gao, S.; Zheng, G., Discovery of a novel (+)-γ-lactamase from Bradyrhizobium japonicum USDA 6 by rational genome mining. Applied and environmental microbiology 2012, 78 (20), 7492-7495. https://journals.asm.org/doi/full/10.1128/AEM.01398-12
Presentations
Invited Talks
12. “Uncovering the Structural and Dynamic Basis of Enhanced Catalysis in Engineered Heme Enzymes Through a Multi-Biophysical Approach”. Next Gen. Conversations, Louisiana Tech University, 2026. Planned.
11. “Protein biophysics and enzyme engineering: bridging fundamental mechanisms with biomedical and chemical applications”, Department of Cell and Molecular Biology, Tulane University. Sep. 12, 2025.
10. “Identification, structural insights, and protein evolution of an enantioselective lactamase for antiviral drug
synthesis", ACS Spring 2024 Meeting, Biochemical Technology Division. March 17-21, 2024. New Orleans, Louisiana, USA.
9. “Protein engineering and structural insights into the lactamase activity and substrate enantioselectivity of a hydrolase from Microbacterium hydrocarbonoxydans”, Department of Biochemistry and Molecular Biology, School of Medicine, Tulane University. Jan 22, 2024.
8. “Identification, structural insights, and protein evolution of an enantioselective lactamase for antiviral drug synthesis", Tulane Center of Biomedical Informatics and Genomics, School of Medicine, Tulane University. Sep. 6, 2023.
Conference Talks
7. “Spatial and Temporal Resolution of Activity-Related Dynamics in the TIM Barrel Enzyme Murine Adenosine Deaminase”, AICHE meetings, Fall 2024, Food, Pharmaceutical & Bioengineering Division, Oct
27-31. San Diego, California, USA.
6. “Hydrogen deuterium exchange within adenosine deaminase provides long range protein networks and structural motifs for the thermal activation of active site chemistry”, ACS Spring 2021 Meeting & Expo, Biological Chemistry Division. April 5-30, 2021.
5. “Identification of Thermal Networks for Catalysis in murine adenosine deaminase”, Rutgers University symposium- Metalloproteins at the Crossroads of Design and Nature, January 27, 2021.
4. “Hydrogen–Deuterium Exchange within Adenosine Deaminase, a TIM Barrel Hydrolase, Identifies 5. Networks for Thermal Activation of Catalysis”, ChemistLive, 2020.
3. “Hydrogen–Deuterium Exchange within Adenosine Deaminase, a TIM Barrel Hydrolase, Identifies Networks for Thermal Activation of Catalysis”, ACS Fall 2020 Meeting & Expo, Biological Chemistry Division.
2. “Mapping Activity-Related Protein Motions in Murine Adenosine Deaminase using Time-, Temperature-, and Mutation- Dependent Hydrogen-Deuterium Exchange Mass Spectrometry (HDX-MS)”, Gordon Research, Seminar, Proteins, Holderness, NH, US, June 16-21, 2019.
1. “Biophysical tools for the detection of protein dynamics across a variety of time scales”, Mainland and Taiwan biotechnology symposium, 2016, Beijing. 1. “Biophysical tools for the detection of protein dynamics across a variety of time scales”, Mainland and Taiwan biotechnology symposium, 2016, Beijing.